Number Of Cysts- The number of cysts decreased significantly in both left (38.2%) and right ovaries (37.28%) on completion of the treatment with Furocyst, whereas in placebo group number of Cysts increased significantly in both left & right ovaries. In Furocyst group, 80% of the patients showed decrease in number of cysts in right ovary and 70.59% of the patients showed decrease in number of cysts in left ovary.
Ovary Volume- The ovary volume decreased significantly in both left (40.12%) and right ovaries (33.05% ) on completion of the treatment with Furocyst, whereas in placebo group ovary volume increased significantly in both left & right ovaries. In Furocyst group, 83.33% of the patients showed decrease in right ovary volume and 73.26 % of the patients showed decrease in number of cysts in left ovary.
Hirsutism Score- There was 27.06% decrease in Hirsutism score in Furocyst treated group, whereas there was a considerable increase in Hirsutism score in placebo treated group. In Furocyst 67.68% of study population showed improvement in Hirsutism score.
Pregnancies – In Furocyst group, total 34 Females got pregnant, 30 during the study and 4 after the completion of treatment.
Prolactin Levels, Free testosterone, LH and FSH level- A significant decrease was observed in Prolactin Hormone levels, free testosterone, LH and FSH Levels with respect to its baseline value on completion of treatment with Furocyst whereas significant increase in Prolactin hormone was observed in placebo group, with no significant change in free testosterone level, LH and FSH level as compared to respective baselines value.
Menstrual Cycle- Furocyst group showed improvement and regularization of menstrual cycle as compared to placebo group. At the baseline, 55% of the study population in Furocyst group showed irregular cycle and 53.6 % of the study population in placebo group showed irregular cycle. The percentage of patients reported irregular menstrual cycle on treatment with Furocyst was reduced significantly and only 13.75% patients reported irregular cycle in the Furocyst group. There was not much improvement in periodicity of menstrual cycle in placebo group. 43.29% of patients still reported irregular cycle at the end of the study treatment. In Furocyst group, 91.25% of the study population showed improvement in menstrual cycle.

Liver Function Test (LFT): No abnormal change in Serum GOT and Serum Alkaline phosphatase activities was observedon treatment with Furocyst and placebo on completion of the respective treatments.
Renal Function test (RFT): No abnormal change in Serum urea and Serum creatinine levels were observed on treatment with Furocyst and placebo on completion of the respective treatments.
Hemogram: No abnormal alteration in hematological parameters were observed on treatment with Furocyst and placebo on completion of the respective treatments.
Lipid profile: There was significant decrease in lipid parameters including cholesterol levels, LDL cholesterol and triglyceride levels of the study population in Furocyst treated patients on completion of the treatment whereas there was no significant alteration in lipid parameters of placebo group.
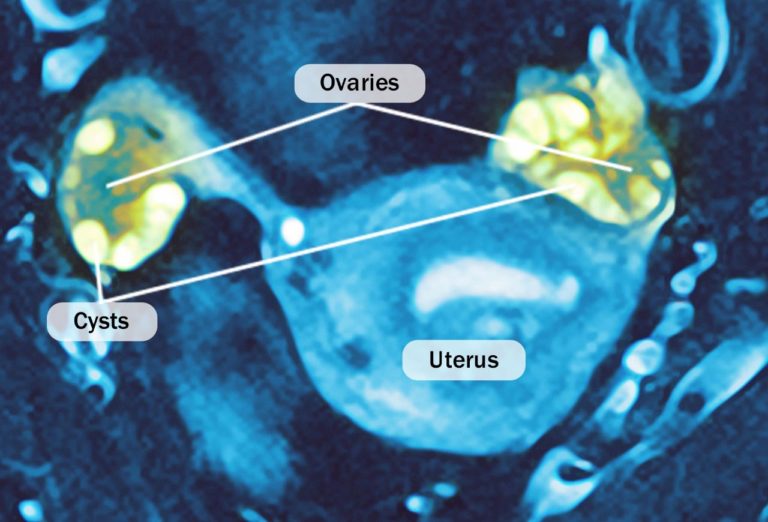
1. Fenugreek seed extract caused a significant reduction in the ovary volume.
6. Overall 94% of the patients reported positively or got benefitted from the fenugreek extract dosing.
Information on this website has been posted in public interest for creating awareness about general health. In event of any copyright conflicts, please inform so that we may take appropriate remedial measures. The products and the claims made on this site have not been evaluated by FDA and are not approved to diagnose, treat, cure or prevent diseases.
Copyright @ 2021 – All rights reserved Chemical Resources.
